working to make science better For the 118 indvidual WIS elements, GO to MORE in the menu above . . .






Helium He
Where is helium used--or where does it impact our everyday lives?
Used in airships--for lighter-than-air flight
Used in transport balloons--see WIS students performing in the National Air and Space Museum's electronic field trip with balloons, including helium balloons, in the Albuquerque Fiesta of Color: see a clip from the episode called Sky Full of Colour
And more of WIS Middle Schoolers in the STEM in 30 program. See all of the program with WIS students A Sky Full of Color, too, on the NASM website
Happy birthday, congratulations, thank you, halloween and many more reasons to celebrate with helium balloons.
The density of He is 0.17 g/dm^3 (g/L, 0.00017 g/cm^3) at room temperature,
air is 1.23 g/dm^3 (1.25 g/L, 0.00125 g/cm^3)
[In comparison, water's density is 1000 g/dm^3 (1000 g/L, 1 g/cm^3)]
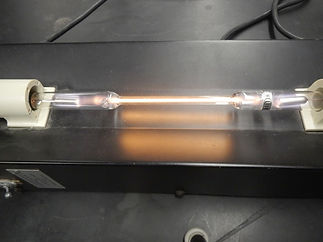
A spectral tube is a sealed glass tube containing a gas at a low pressure and a high electrical voltage is passed through it. The electrical energy causes electrons to be elevated to an excited state--electrons go to a higher emergy level. As they return to the ground state, their base level, energy is emitted. The specific wavelengths of energy produce a particular color.
Helium has particular wavelengths of 447 nm towards the blue end of the spectrum, 501, 587 nm in the orange portion of the spectrum. See the colored spectrum through the diffraction grating. This was investigated by Grade 11 IB Chemistry students.
WIS photographer
The image of the cloud chamber to the left, shows the wispy tracks of condensed ethanol vapor--condensed on a stream of alpha particles. Alpha particles are helium nuclei formed here from the radiactive decay of thorium in the white material poking through the glass dish on the left of the tracks. There is thorium nitrate in the gas mantle.
22890Th --> 42He + 22488Ra
With thanks to Zamam K-E for asking great questions.
WIS Photographer
Helium is an unreactive gas. Cylinders of the gas are used anywhere helium balloons are sold. The cylinders here were photographed where we get our liquid nitrogen for Chemistry Two investigations into gases, where we investigate a mole of nitrogen, the effect of cooling on balloons--including liquefying the air in a balloon and cooling/freezing marshmallows (delicious!), etc.
Below are the enormous helium balloons of the 2015 Macy's Thanksgiving Day parade: Thomas the tank Engine, Angry Bird and a dragon.
Photographed by Annabelle F.



Ms. Redding on a teacher errand involving a helium balloon--we may find out what it will be used for!
She found Nemo!
[Chem would have found a good use for the balloon in during Chemistry TWO Gas Law studies--what volume would the balloon have at different altitudes in the Earth's atmosphere . . . always exciting things to do with balloons


All electronic signs are called "neon" but different gases in the disharge tube, where a low pressure gas is exposed to a high voltage, will produce different colors dependent on the nature/type of gas and whether mercury vapor is also present as well as the nature of the glass.
Helium can form both yellowy lights and red/pink--seen here in the "N" of the OPEN sign at the Peking Imperial restaurant in McLean, VA
WIS Photographer

Helium was discovered spectroscopically, where light is split into characteriastic wavelrngths. Here Chemistry One students are studying the spectra of lights and elements.
WIS Photographer
